Michael Mancino, M.D.
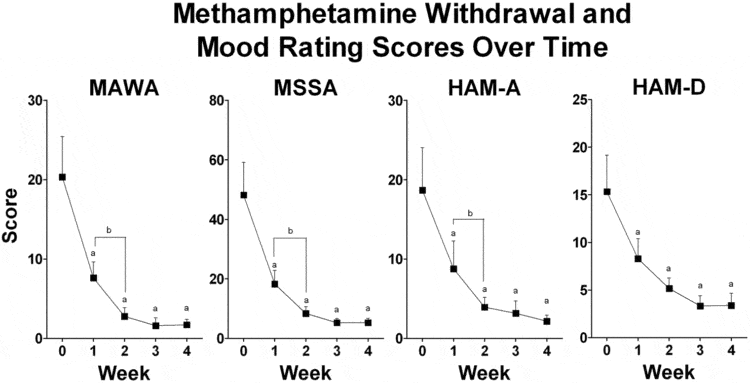
Methamphetamine dependence has become a significant problem, but methamphetamine withdrawal symptoms have not been well studied. Therefore we designed an observational pilot study to examine withdrawal symptoms, mood, anxiety, cognitive function and subjective measures of sleep over a 4-week period in patients entering residential treatment for methamphetamine dependence. Our results demonstrate that methamphetamine withdrawal symptoms, mood and anxiety symptoms all resolve fairly quickly within two weeks of cessation of methamphetamine. However, sleep continued to be disrupted over the course of the 4 week study. No clinically significant alterations in blood pressure or heart rate were identified. This study did not demonstrate any alterations in cognitive function over the 4 weeks of the residential stay.
Selected Publications
Mancino, M.J., Gentry, W.B., Feldman, Z., Mendelson, J.E., and Oliveto, A. Characterizing methamphetamine withdrawal in recently abstinent methamphetamine users: A pilot field study. The American Journal of Drug and Alcohol Abuse. In review.
McGaugh, J., Mancino, M.J., Feldman, Z., Chopra, M.P., Gentry, W.B., Cargile, C., Oliveto, A.O. Open Label Pilot Study of Modafinil for Methamphetamine Dependence. The Journal of Clinical Psychopharmacology. 2009, Oct; 29(5): 488-91.