Primary Investigator: Vladimir Lupashin, Ph.D.
Currently accepting new students
Our laboratory is focused on gaining a deeper understanding of the fundamental principles governing intracellular membrane traffic—a crucial process occurring in every human cell. Intracellular traffic plays a pivotal role in the transportation of proteins and other macromolecules to diverse cellular locations, both within and outside the cell. It serves as a vital mechanism for maintaining cellular homeostasis and significantly influences the interactions between cells and their surrounding environment.
Comprehension of these mechanisms serves as a crucial foundation for developing effective treatments for a wide range of diseases, including but not limited to Congenital Disorders of Glycosylation (CDG), cystic fibrosis, Hermansky-Pudlak syndrome, Parkinson’s, and Alzheimer’s disease. Moreover, it plays a significant role in understanding other conditions such as Amyotrophic Lateral Sclerosis (ALS), and various forms of cancer and diabetes. By unraveling the intricate molecular machinery of intracellular membrane traffic, we aim to uncover novel therapeutic targets and strategies to combat these debilitating illnesses.
Our research team, in collaboration with multiple national and international partners, has played a principal role in the discovery of novel membrane trafficking factors. We contributed over 80 original papers in high-profile journals such as ELife, Journal of Cell Biology, Journal of Neuroscience, Molecular Biology of the Cell, Nature Communications, Nature Optics, Proceedings of the National Academy of Sciences, Scientific Reports, and Traffic. This extensive body of research has garnered continuous support from major funding agencies, including the National Institutes of Health (NIH) and the National Science Foundation (NSF).
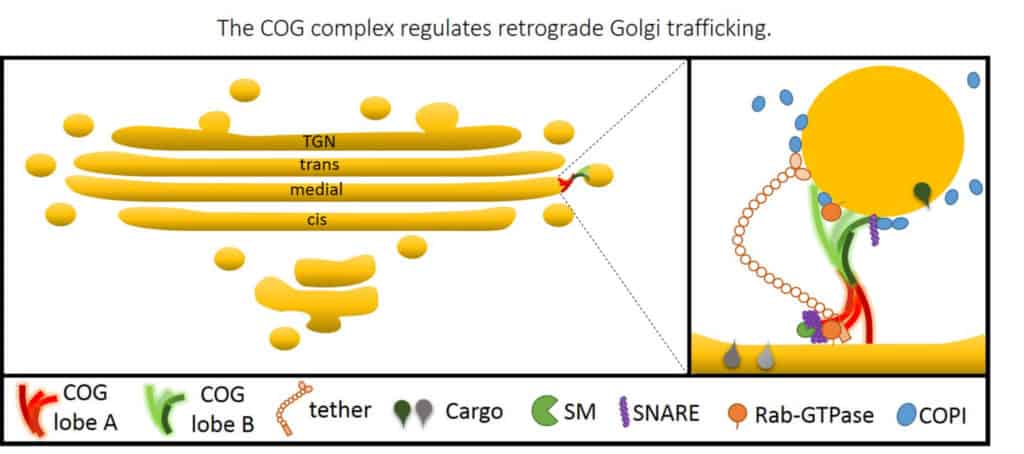
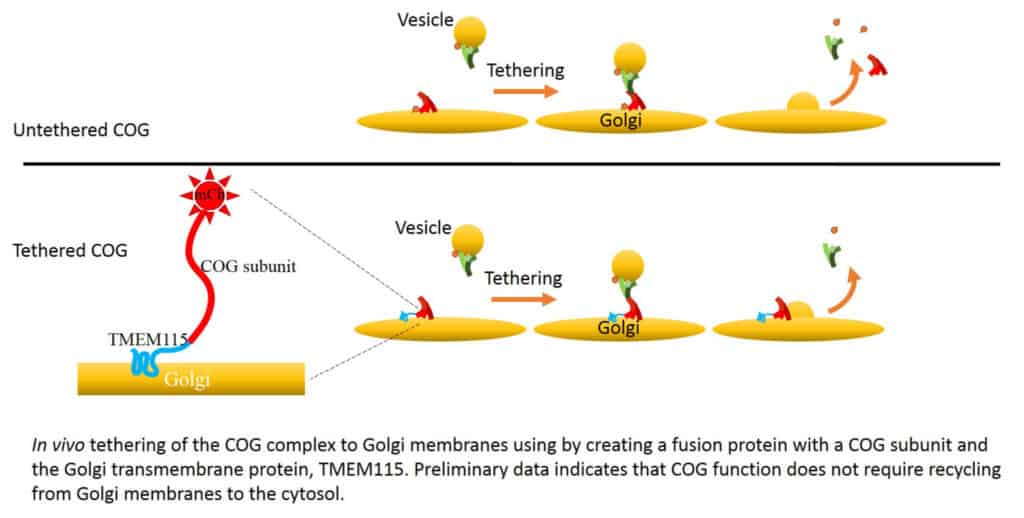
The process of intracellular traffic relies on the coordinated movement of small membrane carriers known as transport vesicles between distinct intracellular destinations. Our laboratory is dedicated to unraveling the intricate molecular machinery that governs the docking and fusion of these vesicles with their intended target compartments. We have pioneered the functional analysis of the Conserved Oligomeric Golgi (COG), an evolutionarily conserved protein complex critical for membrane trafficking and protein modifications in the Golgi apparatus. The Golgi is the central protein modification and sorting station inside the cells. Within this compartment, highly complex sugars or glycans are attached to proteins in the process called glycosylation. The COG complex orchestrates the docking and fusion of Golgi transport vesicles with their acceptor membrane. By using state-of-the-art biochemical (in vitro reconstitution), genetic (CRISPR/Cas9 gene editing), omics (proteomics and glycomics), and microscopy (superresolution fluorescent, live cell, and electron microscopy) approaches, we are deciphering how the key components of intracellular membrane trafficking machinery work together to direct efficient protein trafficking in human cells.
COG mutations disrupt the normal functioning of the Golgi apparatus and proper glycosylation of proteins and lipids, leading to a group of rare diseases known as Congenital Disorders of Glycosylation (CDG). CDGs result in diverse clinical manifestations, including developmental delays, neurological impairments, organ dysfunction, and various other systemic abnormalities. By understanding the impact of COG mutations on Golgi function and glycosylation, we aim to uncover insights into the underlying mechanisms of CDG and develop potential therapeutic interventions to alleviate its associated symptoms.
The COG complex is exploited by numerous pathogens such as viruses (SARS-Cov-2, HIV, Chikungunya, Hepatitis C, Dengue, and Orthopoxvirus), bacteria (Chlamydia and Brucella), and toxins (typhoid, SubAB, Shiga, Ricin, and Cholera). However, the precise mechanisms by which these diverse groups of pathogens evolved to depend on COG function remain an enigma. Understanding the molecular basis of COG-pathogen interactions will allow us to develop strategies to protect cells and organisms from pathogen-borne diseases.
Lab Members
Tetyana Kudlyk, M.S.
Irina Pokrovskaya, M.S.
Zinia D’Souza
Amrita Khakurel
Farhana Sumya
Former Lab Members
Jessica Blackburn
Leslie Climer
Pierre Fotso
Galimat Khaidakova
Svetlana Kononova
Oleksandra Pavliv
Jacob Seiter
Anna Shestakova
Richard Smith
Elena Suvorova
Wei Wang
Rose Willett
Sergey Zolov
Collaborators
Juan Bonifacino (NIH)
Jean Celli (WSU)
Rainer Duden (U. of Lubeck)
Victor Faundez (Emory University)
Francois Foulquier (U of Lille)
Hudson Freeze (Burnham Institute)
Fred Hughson (Princeton)
Willy Morelle (University of Lille)
Sébastien Pfeffer (U of Strasburg)
Kristian Prydz (University of Oslo)
Brian Storrie (UAMS)
Daniel Ungar (University of York)
Representative Publications
Prydz K, Lupashin V, Wang Y, Saraste J. Editorial: Golgi Dynamics in Physiological and Pathological Conditions. Front Cell Dev Biol. doi: 10.3389/fcell.2020.00007 PMCID: PMC7000357
Realegeno, S.; Priyamvada, L.; Kumar, A.; Blackburn, J.B.; Hartloge, C.; Puschnik, A.S.; Sambhara, S.; Olson, V.A.; Carette, J.E.; Lupashin, V.; Satheshkumar, P.S. Conserved Oligomeric Golgi (COG) Complex Proteins Facilitate Orthopoxvirus Entry, Fusion and Spread. Viruses 2020, 12, 707
Blackburn JB, D’Souza Z, Lupashin VV. Maintaining order: COG complex controls Golgi trafficking, processing, and sorting. FEBS Lett. 2019 Aug 5. doi:10.1002/1873-3468.13570. [Epub ahead of print] Review. PubMed PMID: 31381138.
D’Souza Z, Blackburn JB, Kudlyk T, Pokrovskaya ID, Lupashin VV. Defects in COG Mediated Golgi Trafficking Alter Endo-Lysosomal System in Human Cells. Front Cell Dev Biol. 2019 Jul 3;7:118. doi: 10.3389/fcell.2019.00118. eCollection 2019. PubMed PMID: 31334232; PubMed Central PMCID: PMC6616090.
Miller CN, Smith EP, Knodler LA, Cundiff JA, Blackburn JB, Lupashin V, Celli J. A Brucella Type IV effector remodels COG-dependent secretory traffic to promote intracellular replication. Cell Host Microbe. 2017 Sep 13;22(3):317-329.e7.
Comstra SH, Zlatic SA, Gokhale A, Blackburn JB, Werner E, McArthy J, Petris M, D’Souza P, Panuwet P, Boyd Barr D, Lupashin V, Vrailas-Mortimer A, Faundez V. The Interactome of the Copper Transporter ATP7A Belongs to a Network of Neurodevelopmental and Neurodegeneration Factors. ELife 2017 Mar 29;6. pii: e24722. doi: 10.7554/eLife.24722.
Siegel N, Lupashin V, Storrie B, Brooker G. High-magnification super-resolution FINCH microscopy using birefringent crystal lens interferometers. Nature Photonics 2016; 10 (12), 802-808
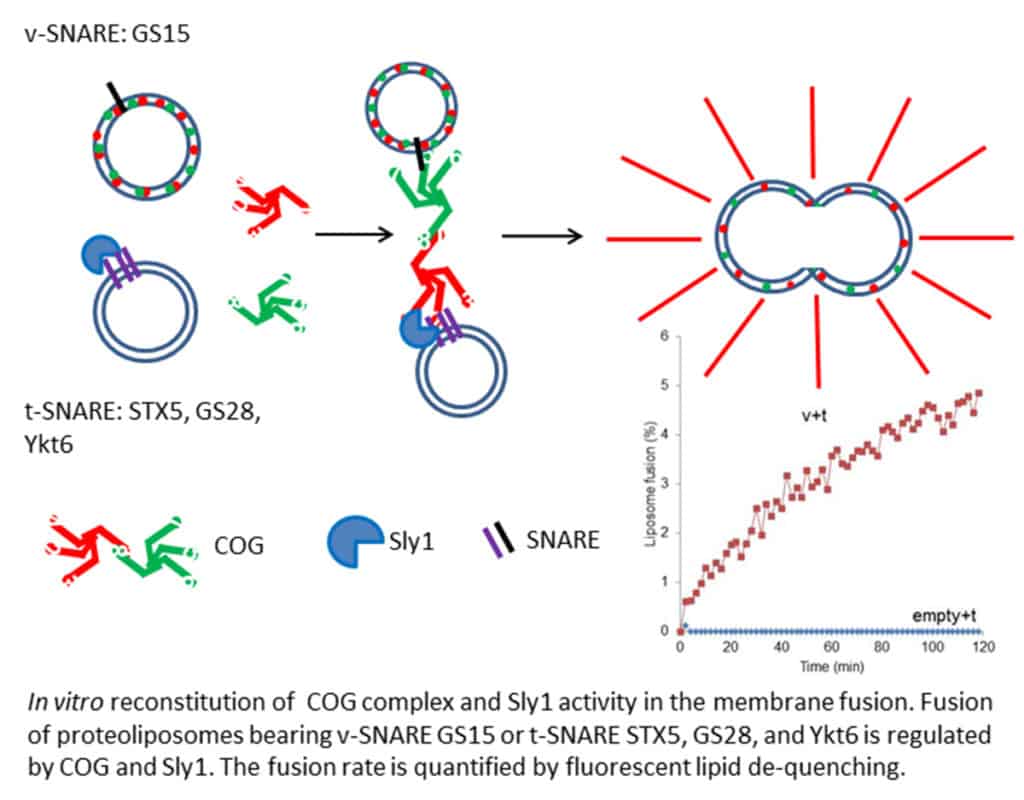
Willett R, Bailey J, Climer L, Pokrovskaya I, Kudlyk T, Wang W, Lupashin VV. COG lobe B sub-complex engages v-SNARE GS15 and functions via regulated interaction with lobe A sub-complex. Scientific Reports 2016; 6; 29139
Climer LK, Dobretsov M, Lupashin V. Defects in the COG complex and COG-related trafficking regulators affect neuronal Golgi function. Frontiers in Neuroscience 2015; 9, 405.
Willett R, Kudlyk T, Pokrovskaya I, Schonherr R, Ungar D, Duden R, Lupashin VV. COG complexes form spatial landmarks for distinct SNARE complexes. Nature Communications 2013, 4:1553
Richardson BC, Smith RD, Ungar D, Nakamura A, Jeffrey PD, Lupashin VV, Hughson FM. Structural basis for a human glycosylation disorder caused by mutation of the COG4 gene. Proceedings of National Academy of Sciences USA 2009;106(32):13329-13334
Zolov SN, Lupashin VV. Cog3p depletion blocks vesicle-mediated Golgi retrograde trafficking in HeLa cells. Journal of Cell Biology 2005;168(5):747-759.