Associate Professor
Ph.D. University of Brescia
Secondary appointments:
Division of Genetics, Department of Orthopaedic Surgery
Office: 501-526-4090
Lab: 501-526-4091
Email: RMorello@uams.edu
Currently accepting new students
Research Interests
In my laboratory, we study the function of novel genes, in particular those involved in connective tissue formation, development, homeostasis, and disease, with an emphasis on the skeleton. We utilize the power of mouse gene targeting and conditional gene-inactivation techniques to generate ubiquitous or tissue-specific mutations in the mouse. With the use of cell biology, biochemistry, cell microscopy, proteomic and genetic approaches we characterize the phenotype of genetically modified mice to understand the underlying gene function. The objective is to learn from the animal model and make correlations with relevant aspects of human disease and hence gain mechanistic insights of biological function.
In former studies, we characterized the function of the Crtap gene, a member of the Leprecan family of genes. Crtap knock-out mice have dramatic low bone mass and a functional defect in bone-forming cells, the osteoblasts. We demonstrated that Crtap forms a complex in the endoplasmic reticulum with two proteins that have an enzymatic function and such trimeric complex has essential collagen post-translational modification and chaperone activity. Importantly, while type I collagen mutations in humans cause autosomal dominant Osteogenesis imperfecta (OI – brittle bone disease), we identified CRTAP as the first gene whose mutations cause recessive forms of OI and this led the way to the identification of several new genes causing similar forms of recessive OI. The study of OI pathogenesis utilizing multiple mouse models is actively pursued in my laboratory.
More recent studies, funded by the NIH, aimed at the characterization of another member of the Leprecan family of proteins, Synaptonemal Complex 65 (Sc65, aka P3H4). This protein is highly similar and evolutionarily related to Crtap but it has been poorly studied. With the hypothesis that Sc65 may have retained a similar function to Crtap, we generated two mouse models of Sc65 loss of function and showed that the loss of Sc65 caused low bone mass and skin fragility. Moreover, we also demonstrated that Sc65 is part of a newly described complex in the endoplasmic reticulum together with P3h3 (prolyl 3-hydroxylase 3) and lysyl-hydroxylase 1 (Lh1). This complex is responsible for the hydroxylation of certain collagen triple-helical lysyl residues that are essential for fibrillar collagen cross-linking in the extracellular matrix. We also showed, in collaboration with others, that loss of Sc65 or P3h3 in the mouse phenocopies the loss of Lh1 and hence these mice represent models of the human disease known as Ehlers-Danlos type VIa.
Another ongoing interest in the lab is the study of osteocytes in osteogenesis imperfecta and their potential role in driving high bone turnover. To do this we deleted RANKL expression specifically in osteocytes in the oim/oim mouse model of OI and are preparing a manuscript to describe our findings.
We also have an interest in the role of collagens and collagen-modifying proteins in other tissues, including the lung and the reproductive system and are doing some work also in these directions.
Besides my departmental colleagues, I have close ties with other faculty on campus that work on bone, including Drs. Manolagas, Jilka, Weinstein, O’Brien, Almeida, Zhao, Porter, Iyer, Ambrogini, Ferreira, Xiong and others and have weekly joint meeting with them to discuss ongoing projects and present our work. We also collaborate with other bone and non-bone researchers on campus as well as with colleagues in the US and the rest of the world.
Teaching Activities
I currently teach muscle and bone physiology within the Musculoskeletal and Skin Block (MSKSK) to the second-year medical students and to the graduate students within the General Physiology course (PHYO 5103).
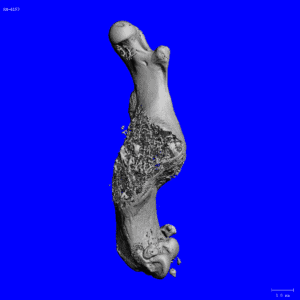
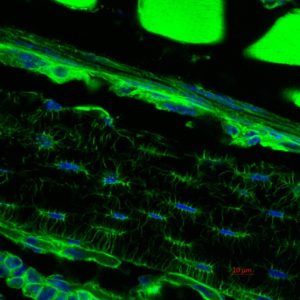
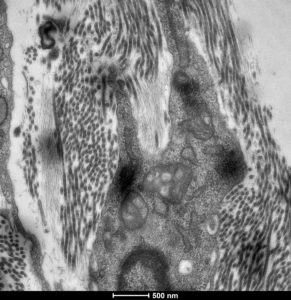
Representative Publications
Hudson DM, Weis MA, Rai J, Joeng KS, Dimori M, Lee BH, Morello R, Eyre DR: “P3h3-null and Sc65-null mice phenocopy the collagen lysine under-hydroxylation and cross-linking abnormality of Ehlers-Danlos Syndrome Type VIA”. J Biol Chem. Mar 3;292(9):3877-3887, 2017.
Morello R: “Osteogenesis Imperfecta and Therapeutics”. Matrix Biol. Oct; 71-72, 294-312, 2018.
Zimmerman SM, Dimori M, Heard-Lipsmeyer ME, Morello R: “The osteocyte transcriptome is extensively dysregulated in mouse models of osteogenesis imperfecta”. JBMR Plus 3(7), 2019, (https://doi.org/10.1002/jbm4.10171).
Dimori M, Heard-Lipsmeyer ME, Byrum SD, Mackintosh SG, Kurten RC, Carroll JL, Morello R: “Respiratory defects in theCrtapKO mouse model of osteogenesis imperfecta”. Am J Physiol – Lung C 318(4): L592-605, 2020 (DOI: 10.1152/ajplung.00313.2019 )
Xu H, Lenhart SA, Chu EY, Chavez MB, Wimer HF, Dimori M, Somerman MJ, Morello R, Foster BL, Hatch NE: “Dental and Craniofacial Defects in the Crtap-/- Mouse Model of osteogenesis imperfecta type VII”. Dev Dyn. 249(7): 884-897, 2020 (https://doi.org/10.1002/dvdy.166).