Mission Statement
- To deliver a diverse, high-quality human biospecimen repository with appropriate patient protections, best practice collection methodologies, clinical data capture mechanisms, and integrated information technology.
- To provide this resource to UAMS research programs to enhance diagnostic, preventative, and therapeutic research.
- To build a self-sustaining, scalable program that focuses and expands resources to meet the needs of researchers.
Services
- Maintain an IRB-approved protocol for the collection and storage of patient specimens.
- Train clinic and research staff to consent patients to the TBAPS protocol before the procedure.
- Collect solid tissue specimens from the pathology grossing room and process (fresh, frozen, FFPE) using standardized protocols.
- Store specimens in vapor-phase isothermal liquid nitrogen tanks or in -80C freezers that are monitored 24/7.
- Track specimen and de-identified pathology information using a secure database with IT oversight and backup. Linkage to identifiable personal health information available with IRB approval.
Using TBAPS
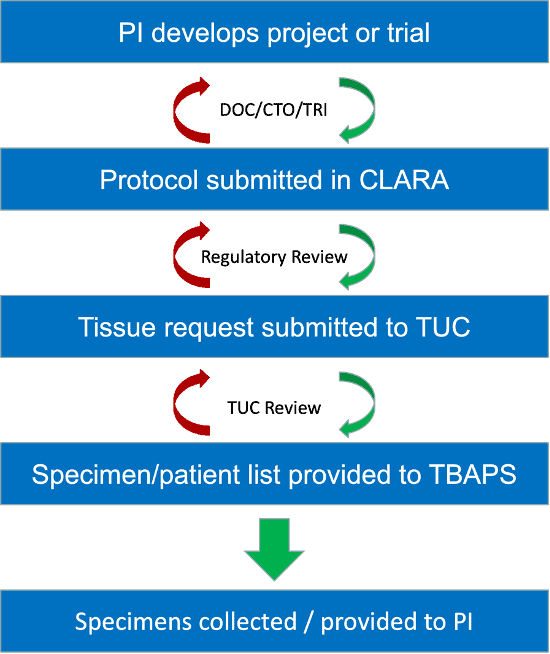
TUC = Tissue Use Committee
Accessible Version of Using TBAPS Graphic
Graphic includes text:
- PI Develops Project or Trial
- DOC/CTO/TRI
- Protocol submitted in CLARA
- Regulatory Review
- TIssue Request submitted to TUC
- TUC Review
- Specimen/patient list provided to TBAPS
- Specimens collected/provided to PI
Researcher Responsibilities
- Abide by the Tissue Use Agreement and terms of the IRB protocol.
- Inform UAMS Tissue Procurement Facility personnel of publications/grants resulting from the use of specimens issued by the UAMS Tissue Procurement Facility.
- Cite the UAMS Tissue Procurement Facility in publications/grants resulting from the use of specimens issued by the UAMS Tissue Procurement Facility.
Frequently Asked Questions
Who has access to donated specimens?
UAMS researchers with an IRB-approved protocol may obtain TBAPS specimens.
Why should I use TBAPS to obtain tissue specimens for my research?
The collection and use of patient samples and data in research are governed by several federal, state, and institutional laws/policies/practices. Using TBAPS ensures compliance with current policies and that samples have appropriate consent for the designated research.
Who owns the specimens stored in the facility?
All tissue and clinical data belong to the patient and the UAMS, represented by TBAPS.
Can donated specimens be withdrawn from the repository?
Yes. Specimens can be withdrawn if a donor withdraws consent or tissue is necessary for further clinical evaluation.
When should I contact TBAPS about collecting or obtaining specimens for my research?
It is best to work with all approving groups as early as possible.
- Is the tissue available?
- What is the collection workflow?
- Are resources available?
Contacts
Remelle Eggerson, B.S.
Tissue Bank Manager
Cancer Institute Walker Tower, 407
Phone: 501-686-7470
Email: EggersonRemelleM@uams.edu
Mindy Gibbons, B.S.N., RNP
Clinical Services Manager
Phone: 501-686-8569
Email: GibbonsMindyG@uams.edu
Director:
Steven Post, Ph.D.
Email: SPost@uams.edu