Assistant Professor
Research Interest: Pathogenesis of leishmaniasis
Ph.D.: University of Georgia, Athens, GA
Postdoctoral Fellowships:
University of Lausanne, Lausanne, VD, Switzerland
University of Pennsylvania, Philadelphia, PA
Phone: 501-686-5518
Fax: 501-686-5359
Email: tweinkopff@uams.edu
Research Description
According to the CDC, more than one billion people, or one-sixth of the world’s population, is suffering from one or more Neglected Tropical Diseases with many of these diseases affecting the poorest populations in the developing world. Our lab focuses on the parasitic disease from Leishmania infection. Cutaneous leishmaniasis has a wide spectrum of clinical manifestations, ranging from self-healing to chronic debilitating disease. Throughout the world, there are one billion people living in endemic areas at risk of infection. Annually, about one million new cases are detected in tropical and subtropical regions with major foci in Asia and South America. Presently, drugs against the parasite are extremely toxic and there is no vaccine. Control of the parasite is dependent upon the activation of macrophages, leading to production of nitric oxide and reactive oxygen species. However, even when an appropriate adaptive immune response develops and parasites are controlled, cutaneous lesions often persist suggesting the inflammatory response can drive pathology. Therefore, the goal of our lab is to define the factors that control both lesion development and resolution which will be important in developing novel therapies for patients with the disease.
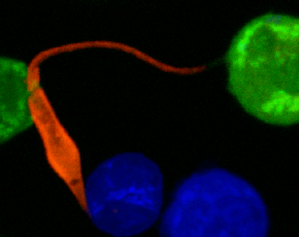
At the site of Leishmania infection, there is a massive infiltration of immune cells and dramatic changes in the vasculature. During infection, new blood vessels form which bring immune cells to the site of infection. Similarly, the lymphatic network also expands to allow these recruited cells to drain back into circulation leading to resolution of the inflammation. In order to characterize the vascular remodeling that occurs during infection, we use a mouse model to manipulate the cells and pathways, such VEGF-A/VEGFR-2 signaling, that are involved in the formation of new vessels. We combine a variety of cellular and molecular techniques, including flow cytometry, microscopy and imaging to address the role of the immune response. Furthermore, we are interested in evaluating the pathways associated with vascular remodeling since they may mediate immune cell infiltration and lesion resolution influencing the severity of disease. We hope the results from our work will provide novel strategies that target the vasculature to reduce the pathology seen in Leishmania patients with non-healing lesions, but also those suffering from other chronic inflammatory diseases and cancer.
Publications
- Bowlin, H. Roys, H. Wanjala, M. Bettadapura, G. Venugopal, J. Surma, M.C. Simon, and T. Weinkopff. Hypoxia inducible factor signaling in macrophages promotes lymphangiogenesis in Leishmania majorinfection. 2021. Infection and Immunity 89 (8): online ahead of print. PMID:34031127.
- Weinkopff*, H. Roys, A. Bowlin, P. Scott*. Leishmania Infection Induces Macrophage VEGF-A Production in a ARNT/HIF-dependent Manner. 2019. Infection and Immunity 87(11): e00088-19. PMCID:PMC6803331. [*indicates co-Corresponding authors]
- Weinkopff, T., C. Konradt, D. Christian, D. Discher, C. Hunter, P. Scott. Leishmania major infection-induced VEGF-A/VEGFR-2 signaling promotes lymphangiogenesis that controls disease. 2016. Journal of Immunology 197(5): 1823-1831. *Article chosen for journal cover image.
- Falcao*, S.A.C., T. Weinkopff*, B.P. Hurrell, F.S. Celes, R.P. Curvelo, D.B. Prates, A. Barral, V.M. Borges, F. Tacchini-Cottier, C.I. de Oliveira. Exposure to Leishmania braziliensis Triggers Neutrophil Activation and Apoptosis. 2015. PLoS NTDs 9(3): e0003601. [*indicates equal contribution]
- Weinkopff, T., C. de Oliveira, A. de Carvalho, Y. Hauyon-La Torre, A. Muniz, J. Miranda, A. Barral, F. Tacchini-Cottier. Repeated Exposure to Lutzomyia intermedia Sand Fly Saliva Induces Local Expression of Interferon-inducible Genes Both at the Site of Injection in Mice and in Human Blood. 2014. PLoS NTDs 8(1): e2627.
- Weinkopff, T., A. Mariotto, G. Simon, Y. Hauyon-La Torre, F. Auderset, S. Schuster, H. Zangger, N. Fasel, A. Barral, F. Tacchini-Cottier. Role of Toll-like Receptor 9 Signaling in Experimental Leishmania braziliensis Infection. 2013. Infection and Immunity 81: 1575-1584.
Dr. Weinkopff’s publications at PubMed.