Assistant Professor
Research Interest: Innate Immunity Defenses Against Intracellular Pathogens
Ph.D.: Indiana State University
Postdoctoral: University of North Carolina at Chapel Hill
Office: 501-686-7428
Lab: 501-320-7706
Fax: 501-686-5359
Email: YAachoui@uams.edu
Currently accepting new students
Research Description
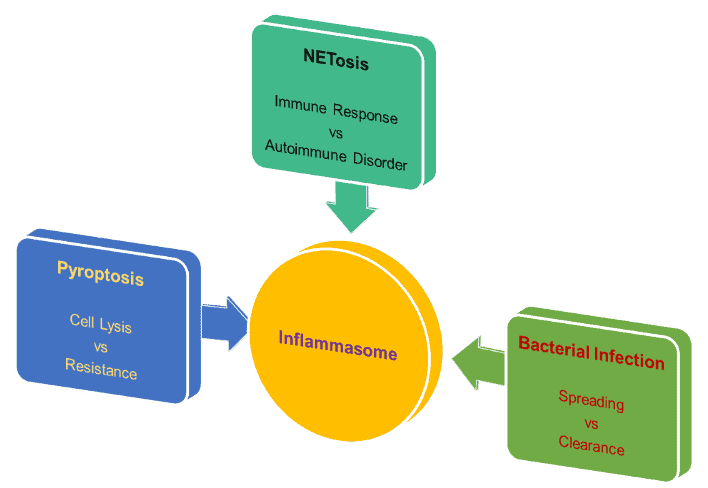
Intracellular bacteria pose a unique challenge to the immune system of mammalian hosts, as they have developed strategies to evade traditional defense mechanisms by residing within host cells. This intricate host-pathogen interaction has led to a complex interplay between immune-sensing and cell death systems. One prominent example of this interplay is observed in the context of inflammasomes and inflammatory caspases.
Traditionally, inflammasomes are responsible for detecting pathogen-associated molecular patterns and activating inflammatory caspase-1 or caspase-11. These caspases play a crucial role in initiating pyroptosis, a form of cell death characterized by the cleavage of the pore-forming protein gasdermin D.
Our research has demonstrated that pyroptosis plays a vital role in restricting the growth of intracellular bacteria. By lysing infected cells, pyroptosis eliminates the intracellular replication niche, exposing the bacteria to attack by neutrophils. We have investigated this phenomenon using various bacterial models, such as Burkholderia and Salmonella.
Furthermore, our studies have revealed that pathogenic bacteria, like Shigella flexneri, employ a bacterial effector called OspC3 to counteract the caspase-11 inflammasome. This finding highlights the sophisticated strategies employed by bacteria to evade immune detection.
More recently, we have made an exciting discovery that neutrophil pyroptosis can even clear extracellular bacteria through inflammasome-mediated neutrophil extracellular traps. This finding expands our understanding of the role of pyroptosis in host defense against bacterial infections.
Our Research Focus
Understanding molecular mechanisms of inflammasome-mediated cell death and physiological relevance:
- Neutrophil resistance to caspase-1-driven pyroptosis
- Pyroptosis-mediated neutrophil extracellular traps for bacterial clearance and autoimmune disorder
- The role of pyroptosis for pathogenic enterobacteria infection and clearance
Team
Changhoon Oh , Ph.D.
Postdoctoral Fellow
Biomed1 Rm 520
Email: COh@uams.edu
Phone (lab): 501-320-7706
• M.S.: Seoul National University
• Ph.D.: Seoul National University
• Postdoctoral Training: UAMS
Amiles Ramos-Etienne
Email: aramosetienne@uams.edu
Phone (lab): 501-320-7706
• B.S.: University of Arkansas at Little Rock (UALR)
Join Us
We are always looking for highly motivated individuals to join our group at all levels. If you have any questions prior to application, please contact Youssef via email at YAachoui@uams.edu.
Recent Publications
- Oh C, Li L, Verma A, Reuven AD, Miao EA, Bliska JB, Aachoui Y. Neutrophil inflammasomes sense the subcellular delivery route of translocated bacterial effectors and toxins. Cell Rep. 2022 Nov 22;41(8):111688.
- Oh C, Verma A, Hafeez M, Hogland B, Aachoui Y. Shigella OspC3 suppresses murine cytosolic LPS sensing. iScience. 2021 Jul 28;24(8):102910.
- Kovacs SB, Oh C, Aachoui Y, Miao EA. Evaluating cytokine production by flow cytometry using brefeldin A in mice. STAR Protoc. 2020 Dec 30;2(1):100244.
- Oh C, Verma A, Aachoui Y. Caspase-11 Non-canonical Inflammasomes in the Lung. Front Immunol. 2020 Aug 21;11:1895.
- Kovacs SB, Oh C, Maltez VI, McGlaughon BD, Verma A, Miao EA, Aachoui Y. Neutrophil Caspase-11 Is Essential to Defend against a Cytosol-Invasive Bacterium. Cell Rep. 2020 Jul 28;32(4):107967.
- Aachoui, Y., and Miao, E.A. (2016). Down with doublespeak: NAIP/NLRC4 inflammasomes get specific. The Journal of Experimental Medicine 213, 646
- Aachoui, Y., Kajiwara, Y., Leaf, Irina A., Mao, D., Ting, Jenny P.Y., Coers, J., Aderem, A., Buxbaum, Joseph D., and Miao, E.A. (2015). Canonical Inflammasomes Drive IFN-β; to Prime Caspase-11 in Defense against a Cytosol-Invasive Bacterium. Cell Host & Microbe 18, 320-332.
- Maltez, Vivien I., Tubbs, Alan L., Cook, Kevin D., Aachoui, Y., Falcone, E.L., Holland, Steven M., Whitmire, Jason K., and Miao, E. A. (2015). Inflammasomes Coordinate Pyroptosis and Natural Killer Cell Cytotoxicity to Clear Infection by a Ubiquitous Environmental Bacterium. Immunity 43, 987-997
- Aachoui Y, Leaf IA, Hagar JA, Fontana MF, Campos CG, Zak DE, Tan MH, Cotter PA, Vance RE, Aderem A, Miao E.A. (2013). Caspase11 protects against bacteria that escape the vacuole. Science. 2013 Feb 22;339 (6122):9758.
- Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao E.A.(2013). Cytoplasmic LPS activates caspase11: implications in TLR4independent endotoxic shock. Science. 2013 Sep 13;341(6151):12503.
- Aachoui Y, Sagulenko V, Miao EA, Stacey KJ. (2013). Inflammasome mediated pyroptotic and apoptotic cell death, and defense against infection. Curr Opin Microbiol. 2013 Jun;16(3):31926.